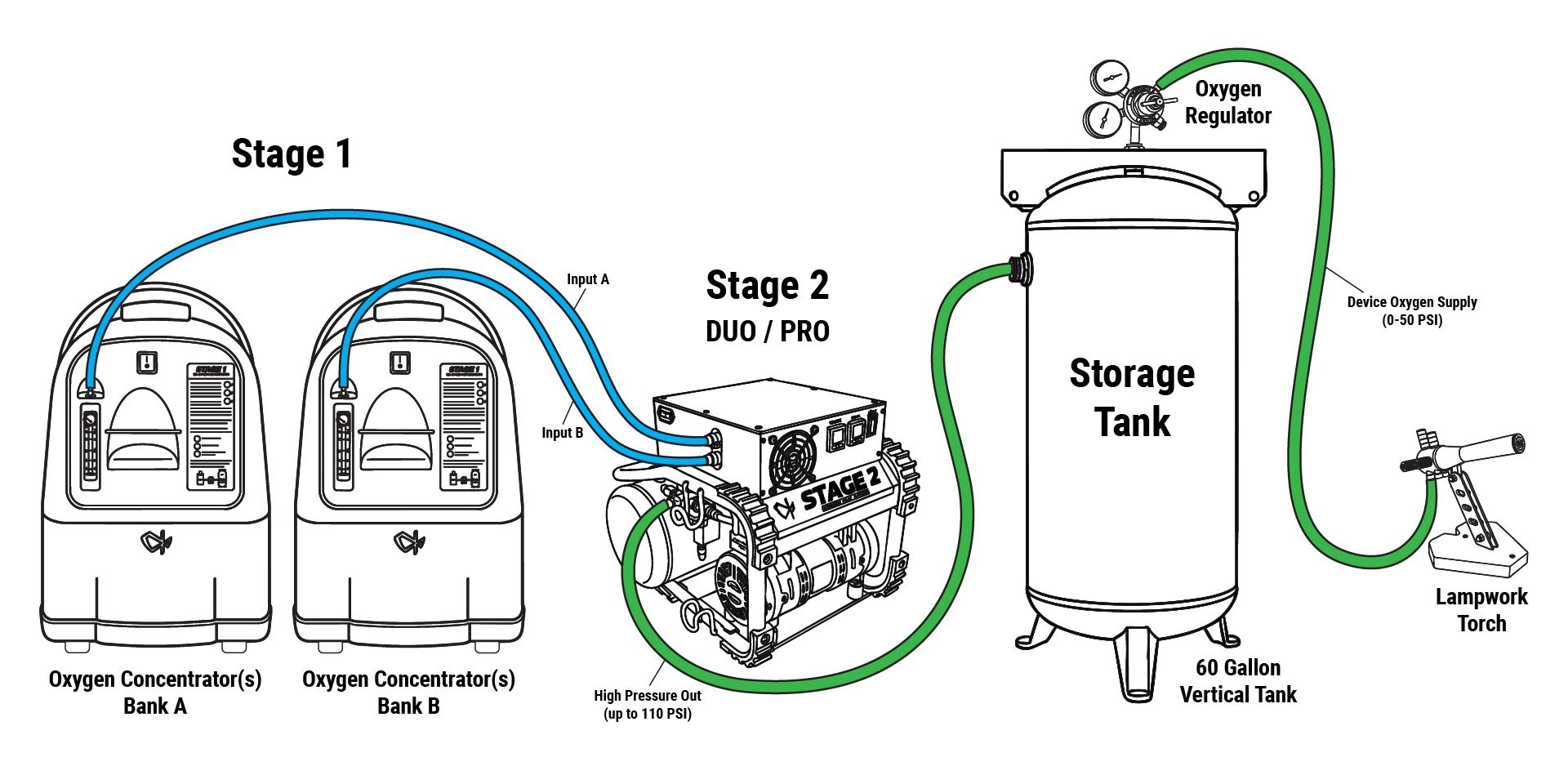
When acquiring a second-hand steel tank for high-purity O₂ service, internal corrosion, residual oils or grease, and flash-rust pose safety and performance risks. This post outlines a validated method for bringing those tanks into service readiness, adapted for oxygen storage applications. It combines degreasing, rust-removal/conversion and proper preparation for subsequent filling.
Why Internal Cleaning Matters
Steel storage tanks that have been used, modified or idle may contain:
- Internal rust or flash rust (even thin iron-oxide layers)
- Residual oils, grease or machining/welding waste
- Moisture or contaminants that could accelerate corrosion or pose ignition or reactivity risks under enriched-oxygen conditions
These issues are especially significant for O₂ service: any hydrocarbon film, moisture or loose scale inside the tank increases hazard potential. Ensuring the internal surface is clean, dry, and in a stable state is key.
Step 1: Degrease & Remove Oils
Before any acid or rust-conversion step, remove oils, grease or organic contaminants. For example:
- Use a degreasing wash such as Simple Green or an industrial-grade alkaline degreaser, fill/flush the tank, agitate, and rinse.
- A coin operated car wash is a good place to gain access to a space to blast an old tank clean.
- Ensure that after degreasing the water rinsing out is clean and that no foaming or oil film remains.
- Dry as much as practical before rust-treatment.
This step matters because residues interfere with later acid conversion, may trap moisture, and under oxygen service could lead to reactivity. In industrial rust-bath literature it is consistently recommended to degrease first. – ChemCafe
Step 2: Rust Conversion / Removal
Once the interior is degreased and rinsed, proceed with a phosphoric-acid based treatment (or a product such as Ospho) to convert or remove internal rust.
Phosphoric Acid Method
Warning: Phosphoric acid can burn skin and eyes and will damage surfaces if spilled. Use proper PPE and mix it safely by adding acid into water. The instructions here are based on common industry practice but may not be suitable for every situation. Always confirm the procedure fits your equipment and follow your local safety and disposal rules. You’re responsible for your own safe handling and use.
- Start with an 85% phosphoric acid – food grade or technical grade ensures there are no unknown variables.
- Mix a working solution: for example a dive-community approach used ~14% acid by volume for moderate scaling; lower concentrations (~2–5%) for light or flash-rust. (These numbers derived from a forum thread on rust cleaning for dive cylinders).
Simple Dilution Guide for 85% Phosphoric Acid
These mixes are designed for 1 gallon of working solution.
Always add acid into water, not the other way around.
Make as many gallons as required for your size tank. We recommend mixing up 1/10th to 1/20th the volume of your tank as phosphoric acid solution.
Heavy Rust / Scale
Purpose: Thick rust, scale, long-neglected tanks.
Strength: Stronger mix.
- Start with 1 gallon of water
- Then add 2 cups of 85% phosphoric acid
This gives a strong solution appropriate for stubborn internal rust.
Moderate Rust
Purpose: Noticeable rust, but not heavily scaled.
Strength: Medium.
- Start with 1 gallon of water
- Then add 1 cup of 85% phosphoric acid
Good all-around cleaning strength for most used tanks.
Light Rust
Purpose: Uniform surface rust, no pitting.
Strength: Mild.
- Start with 1 gallon of water
- Then add ½ cup of 85% phosphoric acid
Great for cleanup after storage or when a tank has a light orange film.
Flash Rust Only
Purpose: Tanks that were clean but flash-rusted during drying.
Strength: Very mild / conversion-only.
- Start with 1 gallon of water
- Then add ¼ cup of 85% phosphoric acid
Application of Phosphoric Acid Solution to Convert and Inhibit Rust
With your solution prepared, proceed with the following:
- Fill the tank partially (e.g., ~1/10th–1/20th of internal volume) with the solution, plug the valves and ports, and rotate/tumble the tank so the solution wets the entire internal surface. Time depends on severity (e.g., 15–30 minutes).
- Drain the solution (capture for reuse or disposal per local regulation).
- Hot-water rinse thoroughly until the tank walls feel warm and the internal surface is well rinsed.
- Dry immediately, using clean, dry, oil-free air (or dry nitrogen) and rotate/roll the tank as drying proceeds. Inverting the tank to blow moisture down can help speed the process up.
- Inspect interior visually (and optionally with borescope) for clean grey/white surface or residual dark phosphate film first
- After drying, install valves and fittings.
- Finally, purge or flush the tank with inert or service gas (e.g., ambient air or N₂) a few times (fill-dump cycles) to remove residual moisture/air before O₂ fill.
Commercial Version of the DIY Phosphoric Acid Treatment: Ospho
Ospho is a commercial rust-converter product that uses phosphoric acid plus wetting-agents and extenders. It converts rust to iron phosphate rather than aggressively dissolving it. Key features:
- According to manufacturer, it “safely dissolves bleeding rust … converts iron oxide to iron phosphate … produces a dry, powdery grey/white surface when cured.” ospho.com
- It is best applied after cleaning off loose scale, dirt, grease.
Step 3: Optional Film-Forming/Corrosion-Inhibitor Compounds

After degreasing and rust conversion/removal, some operators apply a thin film corrosion-inhibitor or amine-based treatment. One compound class worth noting is diethanolamine (DEA / DEOA) — sometimes referenced generically as “Compound O” in industrial metal-processing contexts.
- Diethanolamine is a secondary amine/diol which is used industrially as a corrosion inhibitor and surfactant in metal-working fluids. Third Coast Chemicals
- Its function: it can form a protective amine film on cleaned metal surfaces, reduce re-oxidation (flash rust) during storage, and improve wetting/adhesion of further coatings.
- If used, it must be compatible with oxygen service: the film must be free of oils, solvents or hydrocarbon residues – Compound O is, but not all DEA compounds are.
- The procedure would be: after acid rinse and hot‐dry, apply a very light amine film (in controlled manner), then purge/flush before O₂ filling. Note: you must verify the film does not present an ignition hazard under high partial-pressure O₂ and is compatible with your system’s compatibility standards.
- Because DEA is also flagged with safety/health concerns (it is listed as “possibly carcinogenic to humans” by IARC). Wikipedia
Thus its usage must follow strong safety and compatibility review.
Step 4: Final Drying & Preparation for O₂ Service
Given the service is O₂ storage, the final drying, inspection and inert-purge steps are arguably the most critical.
It’s important to note:
- After cleaning and rinsing, the tank interior must be absolutely dry. Preferably use dry nitrogen or dry shop air that is filtered for oil/moisture and verify dew-point if possible.
- Valve installation should use O₂-cleaned fittings, lubrication, and adhesives (if any) appropriate for O₂ service (no hydrocarbon oils) – Loxeal 85-86 is a great option.
- After valve install, perform a purge/fill dump cycle with inert or ambient gas (e.g., three fill-to-service pressure and dump) to remove any remaining moisture or contaminants.
- A thorough visual inspection with lighting or borescope of the interior surface to confirm no loose scale, no orange rust, no residual film that could react under O₂ service.
- Document the entire preparation: date, tank ID, degreasing solution used, acid concentration, rinse volumes, drying time, purge cycles, inspection findings. This supports traceability and safety efforts.
Summary
By incorporating all four steps — degrease, rust-remove/convert, optional corrosion-film/amine treatment, and final drying/purge — you can bring a second-hand steel tank into a condition suitable for O₂ storage.
Key takeaways:
- Always remove oils/grease first.
- Use phosphoric acid or an equivalent rust-converter (like Ospho) to stabilise rust.
- Consider an amine-based film (e.g., DEA/Compound O) only if fully compatible with O₂ service.
- Drying, purge, inspection and trace documentation are highly suggested for O₂ service safety.
For help with your Oxygen System design and more expert advice, contact us today.